Among different bioprocessing technologies for manufacturing functional recombinant therapeutic proteins (e.g., monoclonal antibody drugs, hormones, and vaccines), yeast-based platforms are among the fastest, most flexible, and economically available to researchers from academia to pharma. Aside from Escherichia coli platforms, which provide several advantages over yeast, including a well-known system, easy workflow, and perhaps the most cost-effective, yeast is undoubtedly the most accessible eukaryotic system that may be leveraged for bioprocessing. A definite advantage over bacterial expression systems is the presence of cellular machinery in the yeast ensuring proper protein folding and the addition of post-translational modifications, such as glycosylation (e.g., N-glycosylation and O-glycosylation), acetylation, amidation, hydroxylation, methylation, phosphorylation, pyrrolidone carboxylic acid, sulfation, and ubiquitylation (Gupta and Shukla, 2017, Gomes et al. 2018).
As a recombinant protein expression system, yeast supports the high-yield production of large proteins (i.e., over 50 kDa), and it’s easily genetically modified by various approaches to optimize metabolic, glycosylation, and secretory pathways, ensuring the generation of functional proteins (Demain and Vaishnav, 2009). Additionally, the development of strains harboring expression cassettes with promoters selected through computational and library screen strategies has optimized protein expression in various yeast variants (Madhavan et al. 2021).
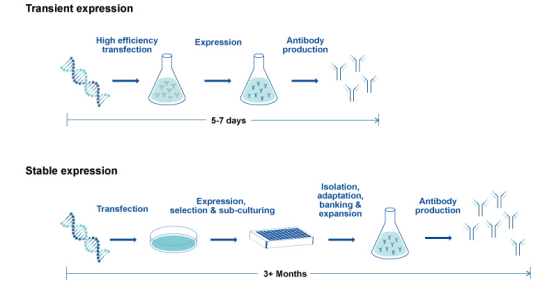 From Transient to Stable CHO Expression
From Transient to Stable CHO Expression
Ultimately, cell line selection for bioprocessing is influenced by factors such as the protein-type being produced and the recombinant protein's glycosylation profile. Significant differences in glycosylation and glycostructure have been reported for proteins produced by CHO vs. HEK293 cells (Croset et al. 2012, Goh & Ng, 2018). Therefore, consistent use of a specific cell expression system across the early and late biotherapeutic protein development stages is critical. For example, the use of CHO transient expression in therapeutic monoclonal antibody development expedites the availability of smaller quantities of antibody candidates, ideal for preliminary in vitro testing. Higher-yield stable CHO expression of lead recombinant monoclonal antibodies is a recommended path towards later preclinical stages. This approach increases the probability that candidates for pharmacological and toxicological characterization conserve established glycosylation patterns supporting IND and therapeutic development (Jain et al. 2017, Sifiniotis et al. 2019).